How does your organization determine calibration test points, tolerances, and frequency? A simple rule of thumb to calibrate instruments at 10, 50, and 90% of full-scale range and use a tolerance of 1.5 or 2 times the manufacturers’ tolerance. Many companies standardize frequencies to calibrate all critical instruments every six months and non-critical instruments once per year. While this approach may make it easy to induct new instruments into the calibration program and manage the calibration schedule, it could be creating extra work and costing you time and money.
In this article, we will discuss using a risk-based approach to determine calibration range, tolerance, frequency, and criticality using the International Society of Pharmaceutical Engineers (ISPE) Good Automated Manufacturing Practice (GAMP) Good Practice Guide, along with tips and tools that can be used for implementation. Following the ISPE GAMP guide will ensure your calibration program meets the Food & Drug Administration’s (FDA’s) promoted initiative of a risk-based approach to Good Manufacturing Practice (GMP) in the 21st Century. This approach places a concentrated focus on risks to product quality and public safety and can often provide cost savings to your organization.
The backbone of such a program depends on making the correct decisions based on reliable, accurate, and traceable information. The required information will be found in User Requirement Specifications (URS), validation protocols, and other documents that were created and approved when the manufacturing process was developed. These documents are used to meet FDA requirements based on the following decisions:
- Whether the drug is safe and effective in its proposed use(s), and whether the benefits of the drug outweigh the risks.
- Whether the drug’s proposed labeling is appropriate and what it should contain
- Whether the methods used in manufacturing the drug and the controls used to maintain the drug’s quality are adequate to preserve the drug’s identity, strength, quality, and purity
Completing risk assessments of your instruments using the above-mentioned documents will determine the proper criticality, calibration frequency, test points, and tolerances based on scientific data, not a rule of thumb or guesswork. It also helps ensure resources are concentrated in the most critical areas of your facility to maximize utilization and reduce cost.
The first step is assembling a team to perform the risk assessments, which should include:
- Process/System Engineer – identify parameters & limits, critical steps in the process, and process tolerances. They will also be responsible for approving the calibration specifications and frequencies and approving the risk assessments.
- Calibration/Metrology Specialists – initiate & manage the risk assessment process, develop the calibration specifications and frequencies, select and approve the appropriate instruments to meet the process requirements, set up the calibration strategy, and approve the risk assessments.
- Quality Assurance – ensure the process parameters and limits meet the regulatory submission, review the calibration specifications and frequencies for compliance with site procedures and the final approval of the risk assessments.
After assembling the team, download a report from your Computer Maintenance Management System (CMMS) that includes the instruments for the process or system you will assess. It will be helpful if the report includes information pertaining to the measurement location in the process/system, the manufacturer and model number of the installed instrument, and the current calibration specifications. The risk assessment process will include answering several questions to determine the correct calibration process, limits, and frequency. Below is an overview of the risk assessment process.
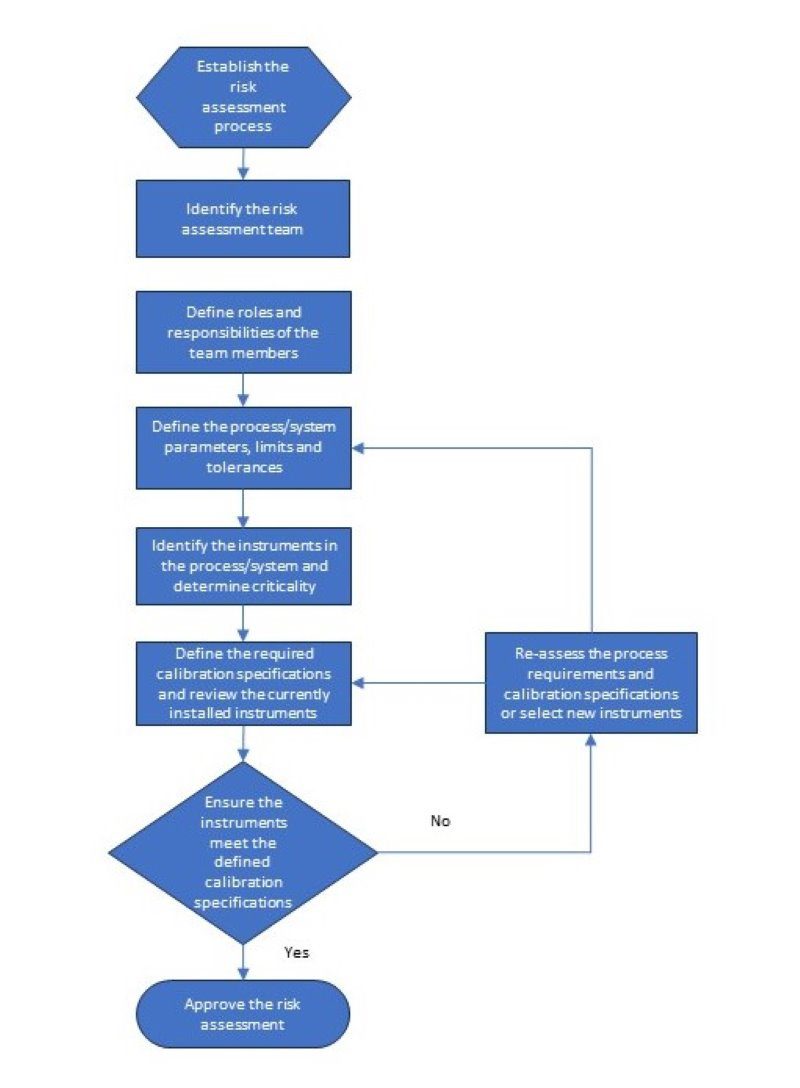
An SOP should be written that details how the risk assessment should be completed and what information is required. Below is a list of the information required to complete a risk assessment, as well as an example assessment process.
- Instrument ID
- Instrument description
- Instrument manufacturer, model & serial number
- Instrument range & accuracy
- What system/process is the instrument installed
- Process range, operating range & tolerance
- Instrument Criticality
- Calibration range, test points & tolerance
- Calibration frequency
- Signatures of the risk assessment team members
Most of the above information comes from the installed instruments and the process documentation. The calibration specifications are determined by answering questions like the ones below.
- Instrument Criticality (may have been previously determined by an impact assessment):
- Is the instrument used for cleaning and/or sterilization of equipment?
- Would a failure impact the product quality or patient safety?
- Would a failure impact the process effectiveness or other business aspect?
- Would a failure create a safety or environmental impact?
If the answer to any of the above questions is “yes,” then the instrument is classified as critical. If the answer to all the questions was “no,” then the instrument is classified as non-critical.
- Calibration range: should be slightly wider than the process range, or alarm range if applicable, to ensure accuracy within the operating range. If an operating range has not been defined or is unknown, calibrating the full range of the instrument is recommended.
- Calibration test points should be at the low and high ends of the calibration range and contain at least one point within the operating range.
- Calibration tolerance should be greater than the manufacturer’s accuracy and less than the process tolerance.
- Calibration frequency may be based on risk factors like these:
-
- If a failure occurs, what’s the impact on product release?
- If a failure occurs, how much product re-work is your company willing to accept?
- If a failure occurs, what’s the environmental impact your company is willing to accept?
- Do you have historical data on-site for the same manufacturer and model instrument?
Through an exercise such as this, your instruments will have increased accuracy and precision throughout the operating range, and completing out-of-tolerance investigations becomes easier now that you have a documented rationale for how the instrument impacts the process. Additionally, most companies determine that some, if not most, of their critical instruments do not need to be calibrated every 6 months. Some companies also opt to extend the calibration frequency of non-critical instruments to 18 to 24 months. Another method used to extend calibration frequency is by using historical data. In this case an initial calibration frequency is set, and after the third calibration is completed a review of the data is performed. If the instrument passes all three calibrations without adjustment, the frequency may then be extended by 50 or 100%. Below is an example of extending the calibration frequency.
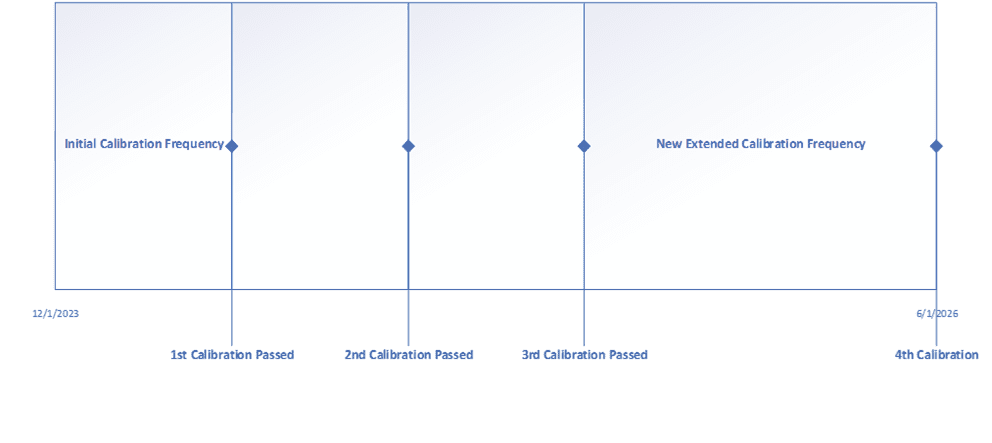
Extending calibration frequencies is where the cost savings will be most noticeable, as less calibration events throughout the year means resources can perform other activities. The important thing to remember is to update any existing procedures that may dictate calibration frequencies.
If your organization would benefit by implementing a risk-based approach to calibration, CAI would like to help. CAI has cross-functional experience in validation, quality, human performance, project management, and maintenance & calibration. Let us become your trusted partner in GMP asset management.
