When it comes to being prepared for an inspection from a regulatory authority, the combination of preparation and practice will often make the difference between success and failure. Regulatory Inspections are becoming increasingly complicated, comprehensive, and higher expectations are required based on the restrictions caused by the COVID 19 pandemic. Manufacturing requirements have become increasingly more complex with the increase in Advanced Therapies (ATMPs) and globalization of the industry. Regulators quickly adapted their inspection processes to provide the necessary oversight of a regulated industry with increasing complex supply chains during a pandemic which prevented significant travel particularly outside of their home country. For routine surveillance/ GMP inspections activities that would traditionally occur on site, the regulators leveraged available tools as well as innovative approaches to support the inspection management program for the fiscal year. Having lived through these virtual inspection processes, the benefits of implementing a digitalized cloud-based inspection management process became clear. Being able to effectively manage, track, and maintain history of inspection activities will raise company quality standards and standardize the inspection process helping to more efficiently meet applicable regulatory and compliance expectations.
Traditional Inspection
Inspections are critical to maintain quality manufacturing. It requires extensive planning, organization, and detailed manual processes and paperwork including procedures, checklists, spreadsheets, facilities, staff, and the training for everyone to use them.. Traditional Inspections follows the process as outlined in the schematic below.
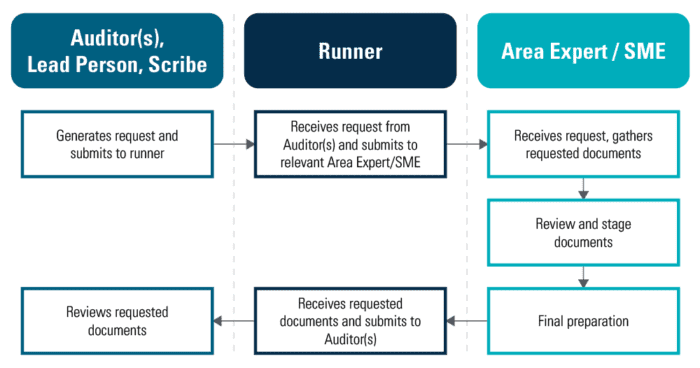
This process becomes even more confusing and stressful when several inspectors send out requests for different documents simultaneously. Tracking of requests, especially urgent ones, becomes confusing and time consuming as documents pile up, no traceability on when or by whom the request was submitted, and general communication breakdowns between the lead in the room with the inspectors, the backroom team, and the area experts ultimately leading to chaos and a delay in timely retrieval of open requests. When there’s no mechanism to organize and manage the chaos, performance suffers.
During an inspection, the organization generally does not stop operations and most Inspectors request to see operations in “full flight”. Maintenance personnel are still checking gauges and preforming routine maintenance activities, operational personnel are busy making product and QC is working to tight timelines to complete in process and finished product testing. It is the responsibility of the audit manager/lead to coordinate the scheduling of key personnel and to effectively organize the audit agenda ensuring the inspection does not impact routine operations. Having an easy to use, effective audit application can relieve these pain points; quality personnel, supervisors, and frontline workers conduct and execute quality audits, identify “near misses”, defects and report on corrective actions in a timely, organized manner. In addition, digitizing the inspection process aids the organization’s sustainability goals and reduces the workload on an already stressed team.
Moving to a digitalized compliance platform can bring improved product quality, reduction in recurring deviations as well as enhanced data security, saving money, resources, and time and ultimately better products to the patient.
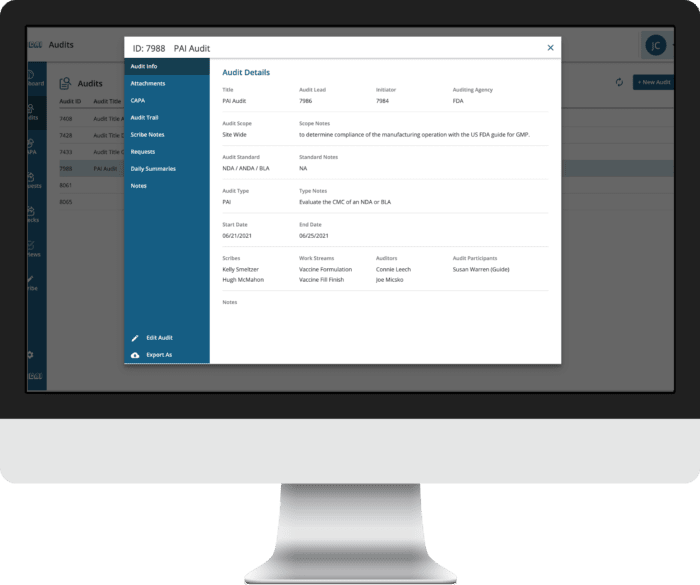 INSPECTION MANAGEMENT SOFT OVERVIEW
INSPECTION MANAGEMENT SOFT OVERVIEW
The CAI Audit App simplifies and manages the complexity of the inspection process easily and intuitively, streamlining the inspection execution by making distressing inspection scenarios a thing of the past. The software is customizable to client’s quality assurance needs and specifications. CAI can help organizations manage and perform inspections, standardize the inspection reporting process, streamline inspection related tasks, and support an audit trail to meet compliance standards. This allows the inspection team to easily track the status of all active, upcoming, and open requests from the user’s dashboard focusing on the inspectors and not document chasing and tracking. Not only does the audit application manage, track, and sort inspection requests instantly, but it also generates reports in real time, summarizes inspection information, and provides charts and graphs empowering the end user to quick identify trends and proactively remediate issues or delays in real time
The application has an industry standard permission based security structure providing for data integrity and seamless integration to the clients information technology allowing system integrators control to control access based on role and job description..
INSPECTION MANAGEMENT SOFTWARE & CONTROL
The CAI Audit App is inspection management software as a service (SasS) that is designed to drive efficiency across your entire audit workflow from planning to reporting. This web-based system provides the Inspection/ Audit manager greater control over the inspection ultimately streamlining the entire inspection process by allowing personnel to track the pace and progress of the audit and better anticipate the inspection needs. It will further minimize the effect of inspections on everyday operations by reducing the number of personnel pulled off operational or core duties and will reduce the amount of time spent on each request whilst maintaining overall efficiency and decreasing personnel stress levels associated with performing the inspection.
The Audit Application manages inspection data in a secure location, generates real-time reports, summarizes inspection information, and enables configurable charts and graphs allowing the user to quickly identify trends.
Key features of the application include:
- Scheduling functionality – rapidly track, sort, and report inspection requests.
- Clearly document the details of the request, track, and record the inspection request information.
- Instantly summarize the status of the inspection at the end of each day
- Easily identify and assign follow-up tasks related to inspection findings in the form of CAPAs.
About the Author:
Connie Leech, Global Director, Quality, Compliance & Regulatory, CAI
Connie has over 15 years of experience in developing, leading, and managing teams of technical and non-technical teams of up to 30 staff including Quality Managers, Quality Engineers, Senior/Junior Quality Specialists, Microbiologists, and QC Analysts. Connie is a Qualified Auditor with experience of FDA/EMA regulatory requirements and inspections applicable to Pharmaceuticals, Biopharmaceuticals, and Medical Device Packaging. She is a practicing QP certifying and releasing finished product in accordance with EU cGMP requirements. Connie is a Quality Leader assuring appropriate life cycle management of the Quality and Compliance systems are developed and implemented to support qualification, technical transfers, regulatory approvals, and ongoing commercial operations.

