Speed to patient is a primary driver behind the industry’s shift toward modern commissioning and qualification (C&Q) practices, while still ensuring product quality and patient safety. The newly revised ASTM E2500-25 standard reaffirms a science- and risk-based approach that not only enhances compliance but also accelerates speed to patient. This optimized, Quality Risk Management (QRM)-based project delivery model offers a powerful solution to address global product shortages and the growing demand for high-quality, life-changing therapies. The use of foundational principles within ASTM E2500 and ISPE Baseline Guide Vol 5 over Multiple projects has already demonstrated tangible success—delivering ahead of schedule and with improved assurance of quality. Now is the time to critically evaluate your current practices and embrace the E2500 framework as a strategic enabler of both operational excellence and regulatory readiness.
What Changed and What is the Impact?
In an era where precision, compliance and agility define pharmaceutical success, the newly revised ASTM E2500-25 standard marks a pivotal shift in how manufacturing systems are specified, designed and verified. This update introduces a science- and risk-based approach that directly aligns system design with process control strategies, ensuring that critical quality attributes (CQAs) and patient safety are safeguarded from the ground up. For manufacturers, this is not just a technical revision—it’s a strategic imperative. Organizations should review and plan to integrate these principles into their commissioning and qualification (C&Q) frameworks, or risk falling behind in regulatory compliance, operational efficiency and product quality assurance.
What’s New in E2500-25?
The 2025 revision of E2500 expands the scope and depth of the standard:
- Introduction of Critical Design Elements (CDEs): These are the building blocks that enable critical aspects of manufacturing systems to consistently control critical process parameters (CPPs).
- Formalization of Design Qualification (DQ): DQ is described to ensure that systems are designed with quality and compliance embedded from the outset. DQ is an early proactive tool to document risk mitigations, formalize design acceptance and empower the verification strategy.
- Expanded Terminology and Roles: Definitions now include Process User Requirements (PURs), General User Requirements (GURs)
,and the System Owner role—each vital for lifecycle accountability.
- System Owner Focus: The System Owner is accountable/responsible for the overall system delivery (Figure 2 below) and is a key single point of contact to ensure continuity and system delivery. The System Owner creates alignment and maintains communication in order to maximize the utility of each phase of system delivery. Should participate in all aspects of system specifications and requirements, as well as risk assessments. The system owner should provide continuity for decisions and history of the system.
- Enhanced Risk Management Integration: Quality Risk Management (QRM) is now embedded throughout the lifecycle, from specification to change control.
- Updated C&Q Delivery Process: See Figure 2 from ASTM e2500-25 below.
- Overall process risk assessments (PRA) are conducted early prior to or during the design phase and form a key input into the project delivery and C&Q process.
- Individual system level risk assessments (SRA) are performed in parallel with the URS to document CAs and CDEs, confirm the design and form the initial verification plan. Note: the PRA and the SRAs form the critical link between process variability and associated risk mitigation/controls to product quality and therefore patient safety
- The Design Review, RTM and DQ confirm the design against the URS and proposed risk mitigations within the PRA/SRA and form the basis of the verification plan. This is done early with where each CA/CDE is verified and by whom.
- IOQ can be a formalized look back at the commissioning phase (FAT, SAT, Vendor IOQ, Installation and Operational Verification) and not a repeated testing effort.
- Clarification on the Qualification phase: Qualification should include verification of critical aspects and critical design elements associated with product quality and patient safety, CPPs and the identified control systems. Verification activities are intended to be an integrated process that includes commissioning and qualification activities. The activities performed during commissioning do not need to be reperformed if verified by an SME (external/internal) if adequately tested. It should be noted that critical aspects and critical design elements should have been approved by quality unit, as agreed in the previously performed Risk Assessment, as well as Design Qualification approvals. Therefore, quality unit approval for commissioning documents is not required. Note: The complexity of verification effort should be commensurate with complexity, novelty and suitability for use of the equipment or system.
Impact on Process Control Strategy
The revised standard emphasizes how manufacturers approach process control:
- From Reactive to Proactive: Instead of verifying quality post-installation, the new model ensures that systems are designed to meet CQAs and CPPs from the start.
- Alignment with ICH Guidelines: The standard now explicitly supports ICH guidelines, reinforcing global harmonization and regulatory expectations.
- Lifecycle Continuity: With the System Owner role and continuous improvement mechanisms, the standard promotes sustained control and adaptability.
Why This Matters
Manufacturers who adopt the updated E2500 framework will benefit from:
- Reduced compliance risk
- Streamlined validation efforts
- Improved product quality and patient safety
- Standardization across systems
Call to Action
If you’re involved in pharmaceutical or biopharmaceutical manufacturing, now is the time to:
- Review your current C&Q practices against the new E2500 framework.
- Train cross-functional teams on the updated terminology and lifecycle roles.
- Integrate proactive QRM and DQ into your project planning and execution. Allocate time and resources for these activities to be performed earlier in the project lifecycle.
Assign, empower, and engage your SMEs and System Owners to ensure accountability and alignment and to de-risk overall system delivery.
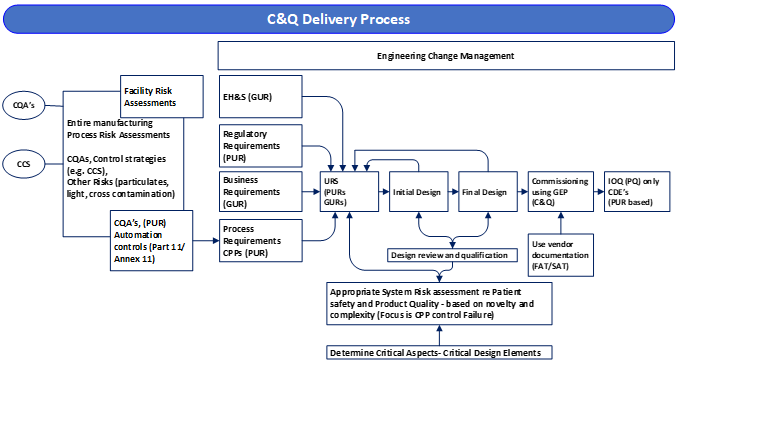
ASTM e2500-25 Figure 2: Commissioning and Qualification Delivery Process
